
Group of synthesis and study of properties of pyrazolotriazinones
Head of research group Oleh Shablykin, Ph.D.
Members of research group:
Artem Hurenko, Ph.D.,
Svetlana Klyuchko, Ph.D.
Publications :
Conversions of 7-aryl-7H-pyrazolo[3,4-d][1,2,3]triazin-4-ons by the action of phosphorus pentoxide, pentasulfide, and oxychloride
B. M. Khutova, S. V. Klyuchko, A. O. Hurenko, A. N. Vasilenko, A. G. Balya, E. B. Rusanov, and V. S. Brovarets
The interaction of 7-aryl-7H-pyrazolo[3,4-d][1,2,3]triazin-4-ols with phosphorus pentoxide or pentasulfide leads to the formation of 6-(5-amino-1-aryl-1H-pyrazol-4-yl)-1-aryl-1H,4H-pyrazolo[3,4 d][1,3]oxazin-4-ones and 6-(5-amino-1-aryl-1H-pyrazol-4-yl)-1-aryl-1H,4H-pyrazolo[3,4 d][1,3]thiazine-4-thiones, respectively.
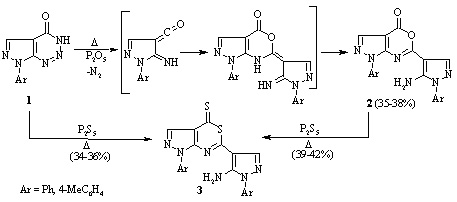
Reaction with phosphorus oxychloride leads
to 1 aryl-5-chloro-1H-pyrazole-4-carbonyl chlorides, from which the corresponding
amides, hydrazides, and substituted 1,3,4-oxadiazoles were synthesized.
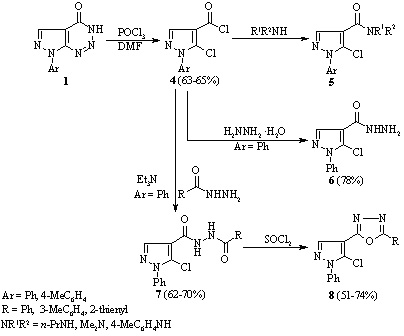
Reaction of 7-phenyl-7H-pyrazolo[3,4-d][1,2,3]triazin-4-ol with thionyl
chloride
B. M. Khutova, S. V. Klyuchko, A. O. Hurenko, A. N. Vasilenko, E. B. Rusanov, and V. S. Brovarets
The reaction of 7-phenyl-7H-pyrazolo[3,4-d][1,2,3]triazin-4-ol with thionyl chloride has been studied in the presence and absence of dimethyl formamide. It was found that heating the compound with thionyl chloride in the absence of dimethyl formamide gave 5-amino-1-phenyl-1H-pyrazole-4-carboxylic acid chloride and in its presence a mixture of 5-chloro-N-formyl-1-phenyl-1H-pyrazole-4-carboxamide, 5-chloro-1-phenyl-1H-pyrazole-4-carboxamide, and 4 chloro-6-(5-chloro-1-phenyl-1H-pyrazol-4-yl)-1-phenyl-1H-pyrazolo[3,4-d]pyrimidine.
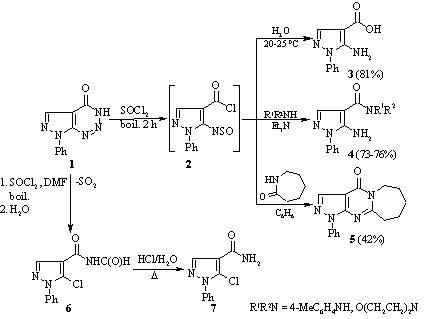
If compound 1 was initially refluxed for 10 min with SOCl2 followed by addition of DMF and refluxing for a further 2 h, the reaction was more complex, and a mixture of products 6, 7 and 4-chloro-6-(5-chloro-1-phenyl-1H-pyrazol-4-yl)-1-phenyl-1H-pyrazolo[3,4 d]pyrimidine 8 was formed, which could be separated by fractional crystallization.
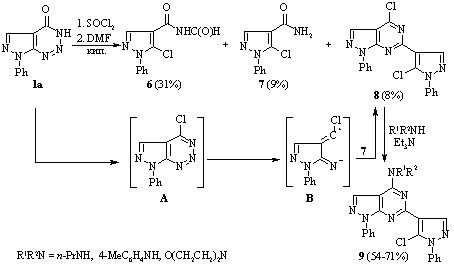
Synthesis of novel pyrazolo[3,4-d][1,2,3]triazines
A. O. Hurenko, B. M. Khutova, S. V. Klyuchko, A. N. Vasilenko, and V. S. Brovarets
Studies have been conducted of the reactions of 7-phenyl-3,7-dihydro-4H-pyrazolo[3,4 d]-[1,2,3]triazin-4-one with alkyl halides and formaldehyde, that occurred with retention of the pyrazolotriazine structure. Ethyl 2-(4-oxo-7-phenyl-4,7-dihydro-3H-pyrazolo[3,4-d][1,2,3]triazin-3-yl)-acetate was prepared by the reaction of 7-phenyl-3,7-dihydro-4H-pyrazolo[3,4-d][1,2,3]triazin-4-one with ethyl chloroacetate. It was used to synthesize the corresponding hydrazide with the aim of introducing the pyrazole or 1,3,4-oxadiazole fragments in the side chain of a bicyclic system.
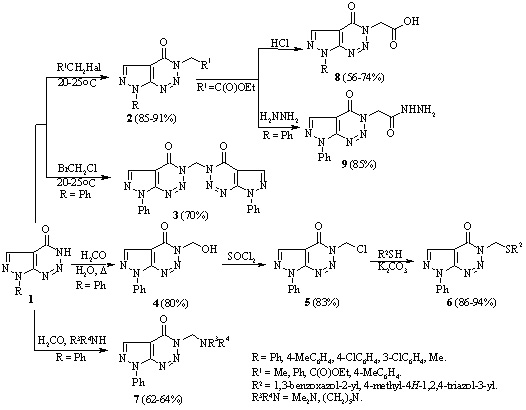
[1] Khutova B. M., Klyuchko S. V., Gurenko A. O., Vasilenko
A. N., Balya A. G., Rusanov E. B., Brovarets V. S. // Conversions of 7-aryl-7H-pyrazolo[3,4-d][1,2,3]triazin-4-ols
by the action of phosphorus pentoxide, pentasulfide, and oxychloride //
Chem. Het. Comp. 2012, Vol. 48, N 8, P. 1251-1262. DOI: 10.1007/s10593-012-1128-6.
[2] Gurenko A. O., Shablykin O. V., Kozachenko A. P., Brovarets V. S.
// Synthesis of the novel heterocyclic system 7,8-dihydroimidazo[1,2-c][1,3]oxazolo[4,5-e][1,2,3]triazine
// Chem. Het. Comp. 2012, Vol. 48, N 9, P. 1423-1424. DOI: 10.1007/s10593
012 1157 1.
[3] Gurenko A. O., Shablykin O. V., Brovarets V. S. // Synthesis of 2-aryl-6H,7H-[1,3]oxazolo[5,4-d]pyrimidine-7-thione
and 2-aryl-6H,7H-[1,3]thiazolo[5,4 d]pyrimidine-7-thione using 2-aroylaminomalonodiamide
// Russ. J. Gen. Chem. 2013, Vol. 83, N 3, P. 572-576. DOI: 10.1134/S1070363213030298.
[4] Khutova B. M., Klyuchko S. V., Gurenko A. O., Vasilenko A. N., Rusanov
E. B., Brovarets V. S. // Reaction of 7-phenyl-7H-pyrazolo[3,4-d][1,2,3]triazin-4-ol
with thionyl chloride // Chem. Het. Comp. 2013, Vol. 49, N 6, P. 922-929.
DOI: 10.1007/s10593-013-1327-9.
[5] Gurenko A. O., Khytova B. M., Klyuchko S. V., Vasilenko O. M., Brovarets
V. S. // Interaction of 5-chloro-1-phenyl-1H-pyrazole-4-carboxamide and
5-chloro-N-formyl-1-phenyl-1H-pyrazole-4-carboxamide with hydrazine hydrate
// J. Org. Pharm. Chem. 2014, Vol. 12, N 1, P. 56-59.
[6] Gurenko A. O., Khutova B. M., Klyuchko S. V., Vasilenko A. N., Brovarets
V. S. // Synthesis of novel pyrazolo[3,4-d][1,2,3]triazines // Chem. Het.
Comp. 2014, Vol. 50, N 4, P. 528-536. DOI: 10.1007/s10593-014-1503-6.
[7] Tsygankova V., Andrusevich Y., Shtompel O., Hurenko A., Solomyannyj
R., Mrug G., Frasinyuk M., Brovarets V. // Stimulating effect of five
and six-membered heterocyclic compounds on seed germination and vegetative
growth of maize (Zea mays L.) // Int. J. Bio. Res. 2016, Vol. 1, Iss 4,
P. 1-14.
© V.P. Kukhar IBOPC NAS Ukraine 2000-2019